News
MASTER DAPT, a landmark trial on abbreviated DAPT post-implantation of Terumo’s UltimasterTM DES in high bleeding risk patients. Final results released @ ESC 2021 and simultaneously published in New England Journal of Medicine and Circulation.
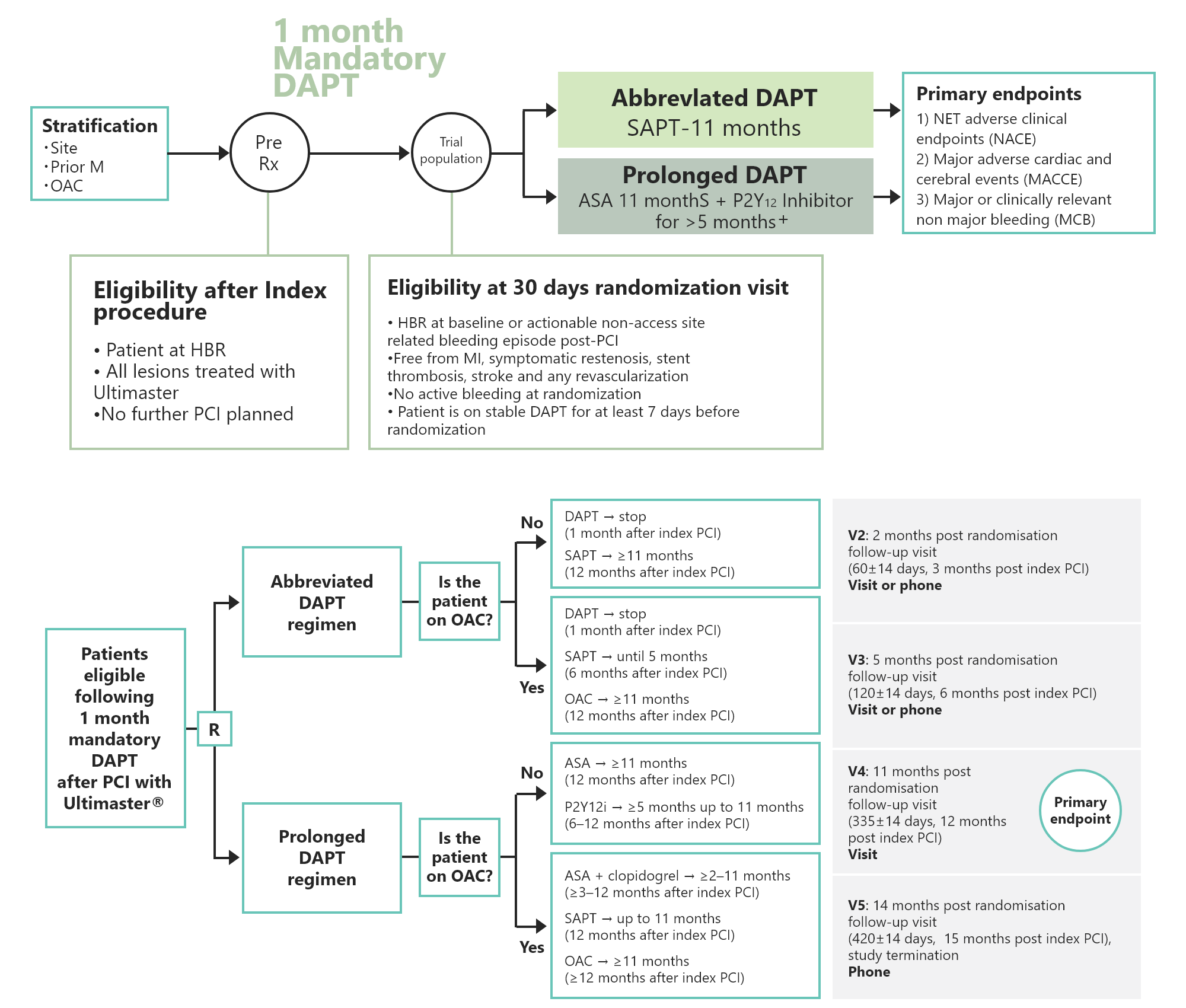
Leuven, Belgium, 2021, August 30th - Terumo announced today the results from the largest multi-center randomized controlled study on the use of short duration of dual antiplatelet therapy (DAPT) in high bleeding risk patients following stenting procedures with UltimasterTM/UltimasterTMTanseiTM drug-eluting bioresorbable polymer stents (DES). The clinical data from this investigator-initiated MASTER DAPT (MAnagement of high bleeding risk patients post bioresorbable polymer coated STEnt implantation with an abbReviated versus prolonged DAPT regimen, NCT03023020)1 study demonstrates that one month of DAPT was non-inferior to treatment continuation for at least 2 additional months for the occurrence of net and major adverse clinical events, and reduced major or clinically relevant non-major bleeding. Randomization was stratified by oral anticoagulation (OAC) indication and the data also demonstrates that abbreviated antiplatelet therapy was associated with similar net and major adverse clinical events but with lower bleeding rates versus non-abbreviated antiplatelet therapy in high bleeding risk patients with or without OAC.
These new clinical data have been released at this years’ European Society of Cardiology (ESC) 2021 congress and have been simultaneously published in New England Journal of Medicine2 and in Circulation3.
A total cohort of 4.579 patients have been randomized into this ambitious study. Patients joined the study from 140 hospitals across 30 countries in Europe, Japan, Asia, Australia and Latin America.
Patients were randomized at 1 month after PCI with implantation of Terumo’s UltimasterTM or UltimasterTM TanseiTM DES to either abbreviated (n=2295) versus prolonged DAPT (n=2284). The patients represent an ‘all-comer’ population, presenting with high bleeding risk features. Randomization was stratified by concomitant OAC indication. At 335 days, complete follow up data were available for 4547 patients (99.3%). An independent clinical events committee blinded to treatment allocation adjudicated all suspected events.
1. Frigoli E et al. Am Heart J 2019;209:97-105
2. M Valgimigli et al. NEJM 2021; DOI: 10.1056/NEJMoa2108749
3. P Smits et al. Circulation 2021; https://doi.org/10.1161/CIRCULATIONAHA.121.056680
S0_Ready to get started? Sign up now!
S0_Lorem ipsum dolor sit amet
S0_2-Col, Right Image
Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut.
Lorem ipsum dolor sit amet, consectetur adipisicing, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut. Ut ad minim veniam.
Vestibulum ante ipsum primis in faucibus orci luctus etel ultrices posuere cubilia Curae.
Primary endpoint
Non-inferiority of abbreviated versus prolonged DAPT for net adverse clinical events (NACE: composite of all-cause death, myocardial infarction, stroke and bleeding events defined as BARC 3 or 5 bleeding events). Non-inferiority of abbreviated versus prolonged DAPT for major adverse cardiac and cerebral events (MACCE: composite of all-cause death, myocardial infarction and stroke). Superiority of abbreviated versus prolonged DAPT for major or clinically relevant non-major bleeding (MCB: composite of type 2, 3 and 5 BARC bleeding events).
The study has 3 primary endpoints:
Comments from Principal Investigators
Principal Investigator Prof Marco Valgimigli, Professor of Cardiology, deputy Chief of Cardiology and Director of Clinical Research, Istituto Cardiocentro Ticino, Lugano, Switzerland said: MASTER DAPT has shown that 1-month DAPT followed by single antiplatelet therapy up to 1 year or 6 months after UltimasterTM stent implantation preserves the ischemic risks while it mitigates the overall bleeding burden in patients who are at high risk for bleeding. This is a ground-breaking new information for clinicians who now know that reducing DAPT duration to 1 month is the new standard of care in this selected high bleeding risk population. These results carry high clinical relevance considering that patients with acute coronary syndrome or who underwent complex or multivessel PCI, including left main stenting were similarly included in our trial.
Principal Investigator Dr Peter Smits, Head of Intervention Cardiology, Maasstad Hospital, Rotterdam, complemented: The OAC - non OAC subgroup analysis clearly shows that with the UltimasterTM stent it is safe and beneficial to stop DAPT after 1 month both in the high bleeding risk populations with or without a clinical indication for oral anticoagulants. This is important additional information, as PCI patients with oral anticoagulants are at very high bleeding risk and little was known about the optimal DAPT duration in these patients.
Toshi Osada, President of Terumo Corporation’s Cardiac and Vascular Company commented: “It is great honor for us to support and be part of such a clinically valuable study. The MASTER DAPT study is a big step to improve high bleeding risk patients’ prognosis. Going forward, we will continue to bring innovation to the field of interventional cardiology by delivering quality products and services, and by supporting to build clinical evidence for the future of healthcare”.
The Principal Investigators concluded: “We thank all the investigators and patients who agreed to participate in this study, helping us meet this major milestone,” the Principle Investigators said. “MASTER DAPT is a truly global effort with hundreds of dedicated stakeholders sharing a vision of advancing care for a large and vulnerable patient population that has been too frequently excluded from major trials.”
A shorter DAPT protocol will save substantial healthcare resources by reducing cost for DAPT, the number of hospitalizations for bleeding, and the number of working days lost.
Product Information
The UltimasterTM and UltimasterTM TanseiTM DES have extensive real-world clinical data, having been studied in a population of over 50,000 patients. Both DES are designed to promote optimal vessel healing and therefore hypothesised to facilitate a shortened DAPT regimen. This hypothesis was confirmed in the DISCOVERY 1TO3 clinical trial that proved an excellent strut coverage as early as 1 month.4 Robust safety data includes published results from the CENTURY II trial that showed a low stent thrombosis rate of 0.2% between 1 and 5 years.5 The efficacy and safety of the stent have been further confirmed by results from the e-ULTIMASTER registry, one of the largest real-world registries, including complex PCI patients.6
4. B. Chevalier et al. Circ Cardiovasc Interv. 201710: e004801
5. Wijns W. et al. EuroIntervention 2018;14:e343-351
6. Mohamed MO et al. EuroIntervention 2020 ;16 : 603-612
S1_Lorem ipsum dolor sit amet, consectetur adipiscing elit
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Ultimaster
Design ensuring better biocompatibility for long-term safety.
・Long term efficacy and safety proven by RCT in patients with complex background.
・Early healing by abluminal gradient coating and reduced drug dose.
・Reliable long-term patency by stronger vessel support and fracture resistance.
Long term efficacy and safety proven by RCT in patients with complex background.
CENTURY II trial is a prospective, multicenter, randomized(1:1), single-blind, controlled, non-inferiority, and two-arm trial comparing Ultimaster and Xience. Complex patient subset results obtained from 5-year follow up shows numerically lower with significant trend.... more
Ultimaster Tansei
Cross challenging anatomy with confidence. Advanced shaft technology for outstanding acute performance. Track complex lesions with ease. A tip designed to facilitate treatment of the most challenging cases.
・Updated exit port - smooth and balanced transition
・Advanced shaft technology - good transmission force and pushability, excellent kink resistance
・Innovative tip - Optimized durability and clear visibility ...more
S1_Ut Enim Minima
Sed ut perspiciatis unde omnis iste natus error sit voluptatem!
Quis Autem Vel
Nemo enim ipsam voluptatem quia voluptas sit odit aut fugit!
Quo Voluptas
Ut enim ad minima veniam, quis nostrum exercitationem ullam!
S1_Lorem ipsum dolor sit amet
References
Enrico Frigoli et al, Design and rationale of the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen (MASTER DAPT) Study, Am Heart J. 2019 Mar;209:97-105.
Publication (External) >
S2_2-Col, Right Image
Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut.
Lorem ipsum dolor sit amet, consectetur adipisicing, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut. Ut ad minim veniam.
Vestibulum ante ipsum primis in faucibus orci luctus etel ultrices posuere cubilia Curae.
S2_2-Col, Left Image
Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut.
- Many addon features
- Fully responsive & adaptive
- SEO optimized
- Attractive with a modern touch
- Full Support
S2_Ut Enim Minima
Sed ut perspiciatis unde omnis iste natus error sit voluptatem!
Quis Autem Vel
Nemo enim ipsam voluptatem quia voluptas sit odit aut fugit!
Quo Voluptas
Ut enim ad minima veniam, quis nostrum exercitationem ullam!
S2_Consectetur adipiscing elit...

Joanna C.
"Et harum quidem rerum facilis est et expedita distinctio!"

Stanley T.
"Nam libero tempore, cum soluta nobis est eligendi."

Danielle W.
"Temporibus autem quibusdam et aut officiis debitis!"
S2_Lorem ipsum dolor sit amet
S3_Lorem ipsum dolor sit amet, consectetur adipiscing elit
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
S3_2-Col, Right Image
Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut.
Lorem ipsum dolor sit amet, consectetur adipisicing, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut. Ut ad minim veniam.
Vestibulum ante ipsum primis in faucibus orci luctus etel ultrices posuere cubilia Curae.
S3_2-Col, Left Image
Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut.
- Many addon features
- Fully responsive & adaptive
- SEO optimized
- Attractive with a modern touch
- Full Support
S3_Ut Enim Minima
Sed ut perspiciatis unde omnis iste natus error sit voluptatem!
Quis Autem Vel
Nemo enim ipsam voluptatem quia voluptas sit odit aut fugit!
Quo Voluptas
Ut enim ad minima veniam, quis nostrum exercitationem ullam!
S3_ Consectetur adipiscing elit...

Joanna C.
"Et harum quidem rerum facilis est et expedita distinctio!"

Stanley T.
"Nam libero tempore, cum soluta nobis est eligendi."

Danielle W.
"Temporibus autem quibusdam et aut officiis debitis!"
S3_Lorem ipsum dolor sit amet
S4_2-Col, Right Image
Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut.
Lorem ipsum dolor sit amet, consectetur adipisicing, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut. Ut ad minim veniam.
Vestibulum ante ipsum primis in faucibus orci luctus etel ultrices posuere cubilia Curae.
S4_Lorem ipsum dolor sit amet, consectetur adipiscing elit
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
S4_2-Col, Right Image
Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut.
Lorem ipsum dolor sit amet, consectetur adipisicing, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut. Ut ad minim veniam.
Vestibulum ante ipsum primis in faucibus orci luctus etel ultrices posuere cubilia Curae.